Molecular Mechanisms of West Nile Virus Neuroinvasion
Home
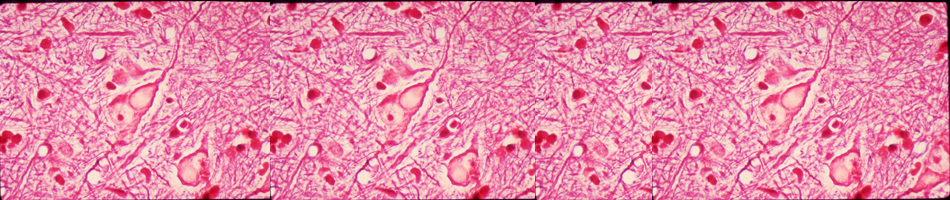
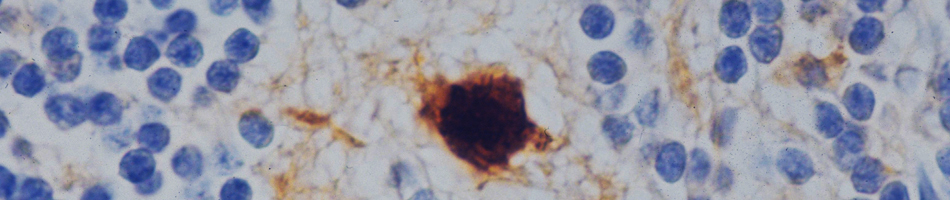
Infection with West Nile virus (WNV), a neurotropic flavivirus, activates neuroinflammatory responses resulting in blood-brain barrier (BBB) disruption and increased infiltration of infected immune cells into the central nervous system (CNS). Although matrix metalloproteinases (MMP) and tissue inhibitors of MMP (TIMP) induced during neuroinflammation have been implicated in the disruption of the BBB, the pathways involved in their induction and their role in monocyte transmigration across the BBB in WNV
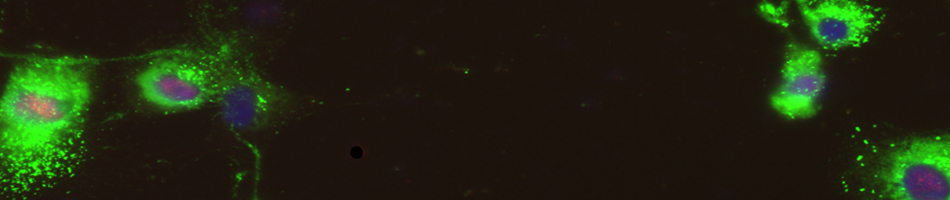
infection are unclear. Our preliminary studies demonstrate significant induction of multiple MMPs in human monocytes and brain cortical astrocytes. Understanding the specific mechanisms involved in BBB disruption is likely to yield novel approaches aimed at increasing survival and improved management of WNV neuroinvasive disease.
Central hypothesis
Increased production of matrix metalloproteinases (MMPs) by WNV-infected monocytes and astrocytes, via the cyclooxygenase-2 (COX-2)-induced urokinase-plasminogen activator (uPA) signaling pathway, mediates the BBB breakdown by degrading specific tight junction proteins (TJP) and allows the unrestricted entry of WNV-infected monocytes into the CNS thereby contributing to WNV neuropathogenesis.
Objective
To characterize the role of uPA and MMPs in BBB opening and trafficking of WNV-infected monocytes into the CNS.
Specific Aim 1
Analyze the role of COX-2-induced urokinase plasminogen activator (uPA) signaling pathway in regulating the expression and activity of MMP in WNV-infected human monocytes and astrocytes by using COX-2 siRNA.
Specific Aim 2
Use a well-established in vitro BBB model and MMP inhibitors to determine if MMP upregulation leads to the degradation of TJP resulting in increased permeability and transmigration of infected monocytes across the BBB model.
Specific Aim 3
Employ a mouse model to characterize and assess the potential of MMP inhibitors to prevent BBB disruption and eventual entry of WNV into the CNS.
Saguna Verma, Ph.D.
Associate Professor
Department of Tropical Medicine, Medical Microbiology and Pharmacology
John A. Burns School of Medicine
Email: saguna [at] hawaii.edu
Katherine E. Conant, M.D.
Research Associate Professor
Department of Neuroscience
Georgetown University, Washington, DC
Email: kec84 [at] georgetown.edu
Kelsey Roe, M.S., Ph.D.
Research Assistant
Department of Tropical Medicine, Medical Microbiology and Pharmacology
John A. Burns School of Medicine
Email: kelsey20 [at] hawaii.edu