Biocontainment Core
Home
Research on microbial agents, which cause lethal diseases in humans and for which effective drugs or preventive vaccines are not available, must be conducted by well-trained investigators in specially built, well-maintained laboratories. The Biocontainment Core provides the triad of customized service, research and development, and education and training to investigators at the university and the wider research community.
Objective
To facilitate the transition of the Biocontainment Core into a sustainable state-of-the-art core facility capable of supporting high-caliber research on new, emerging and re-emerging infectious diseases.
Specific Aim 1
- Enhance and streamline core operations.
Specific Aim 2
- Grow and diversify user base, capability and reach.
Specific Aim 3
- Strengthen and sustain core infrastructure.
Vivek R. Nerurkar, Ph.D.
Director, Biocontainment Core
Professor and Chair
Department of Tropical Medicine, Medical Microbiology and Pharmacology
John A. Burns School of Medicine
Email: nerurkar [at] pbrc.hawaii.edu
Mukesh Kumar, Ph.D.
Associate Director, Biocontainment Core
Assistant Professor
Department of Tropical Medicine, Medical Microbiology and Pharmacology
John A. Burns School of Medicine
Email: mukesh [at] hawaii.edu
Services/Assays
BSL-3 assays and services
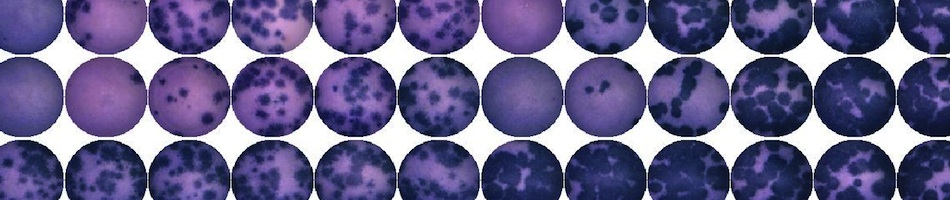
- West Nile Virus plaque assay: Titration of serum samples or tissue homogenates on Vero cells, using traditional agar overlays and neutral red. Calculate virus titers using Excel or your specified output file.
- Homegenate preparation for plaque assay or ELISA: Homogenization of tissue samples using the bead-based Bullet Blender or Fastprep. Clarified samples stored frozen in the facility until used for the plaque assay, ELISA, or other assays.
- West Nile virus PRNT assay: Determination of PRNT90 values for serum samples. Raw data and calculated values returned to the user in Excel or specified output file.
- Luminex assay: Plate set-up, incubation of your samples with beads from your kit, and running of plate on the Luminex 200 instrument in our facility. Raw data files returned in Excel or your specified output file. Samples accepted for testing include: serum, 0.4 microM filtered tissue homogenate, and cell supernatant. Other specimens subject to approval from the Laboratory Director.
- Virus production: Culture, titration, and working dilution determination of West Nile virus or other IBC approved virus. Vials will be stored in our facility until needed for assays.
- Nucleic acid isolation: Isolation of total RNA or genomic DNA for downstream analysis such as RT-PCR. Concentration of each sample determined using UV spectrometry.
- RT-PCR: Plate set up, cDNA synthesis, and running of samples with primers and probes on the real-time PCR instruments. Five different color detection options. Quantitative or relative analysis available.
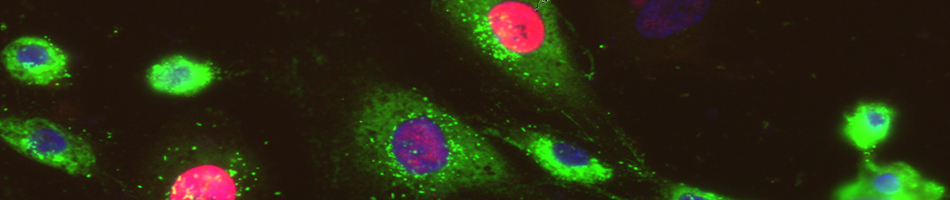
- Flow cytometry: Creation of single cells suspensions from your tissue samples using the Gentle MACS system, and staining of cells with antibodies. Samples will be fixed and run on the FACS Aria flow cytometer. FACS Aria charges are billed separately.
- Freezer rental space: Affordable monthly rentals space on a per box basis.
- Other services: Cell migration assays, cryostat sectioning, fluorescent microscopy, and protein isolation. Please contact us for more information, or if you may require an assay or procedure that are not included in this list. Subject to authorization by the Biocontainment Laboratory Director.
ABSL-3 assays and services
- 21-day survival study: Inoculation of mice with West Nile virus and monitoring of symptoms for three weeks. Includes chain of custody, husbandry, removal of carcasses, and terminal bleed out of survivors.
- Eight-day comparison study: Inoculation of mice with West Nile Virus and monitoring of symptoms for eight days. Includes chain of custody and husbandry.
- Tissue harvest with perfusion and/or paraformaldehyde tissue preservation: Perfusion and collection of blood/serum and specified tissues. Tissues are flash frozen in liquid nitrogen or fixed in formaldehyde and embedded in OCT medium for sectioning. Rates charged are per harvest.
- IP injection: Animals will be administered doses of your IBC/IACUC approved compound via intraperitoneal injection route. Rates charged are per injection.
- Blood collection via tail-bleed: Less than 100 uL of blood will be collected via tail-bleed from mice according to the approved IACUC protocols, and then centrifuged to freeze serum for downstream analysis. Rates charged are per collection.
- Tissue harvest (non-perfused): Collection of blood/serum and specified tissues. Tissues are flash frozen in liquid nitrogen. Rates charged are per harvest.
- Evans Blue Assay: Injection of mice with Evans blue dye for blood-brain barrier permeability assessment. Services include images and scoring of each sample.
- Other services: Intracranial injections and survival surgeries. Please contact us for any services you may require that are not included in this list. Subject to IBC/IACUC approval and authorization by the Biocontainment Laboratory Director.
Training opportunities
Experienced staff will provide hands-on training to investigators in conducting IBC/IACUC approved BSL-3/ABSL-3 research protocols. Please refer to our training document (Training Required for New Personnel) to help you get started on the process for obtaining clearance to work in the JABSOM Biocontainment Facility.
Equipment
Protocols/Procedures
Please fill out the service request form and email it to the ABSL/BSL-3 Biocontainment Core.
Publications
Citations in Publications
Use of this core facility should be acknowledged in publications, abstracts, posters and oral presentations. The suggested verbiage is:
“Some of the services for this research were provided by the ABSL-3/BSL-3 Biocontainment Core, which is supported in part by grant P30GM114737 from the Centers of Biomedical Research Excellence (COBRE) program of the National Institute of General Medical Sciences, a component of the National Institutes of Health.”
2011-2012
- Arai S, Gu SH, Baek LJ, Tabara K, Bennett SN, Oh HS, Takada N, Kang HJ, Tanaka-Taya K, Morikawa S, Okabe N, Yanagihara R, Song JW. (2012) Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012 Jan 7. [PMID: 22230701]
- Huang Z, Hoffmann FW, Fay JD, Hashimoto AC, Chapagain ML, Kaufusi PH, Hoffmann PR. Stimulation of unprimed macrophages with immune complexes triggers a low output of nitric oxide by calcium-dependent neuronal nitric-oxide synthase. Journal of Biological Chemistry 2012 Feb 10;287(7):4492-502. [PMCID: PMC3281602, PMID: 22205701]
- Kang HJ, Bennett SN, Hope AG, Cook JA, Yanagihara R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. Journal of Virology 2011 Aug;85(15):7496-503. [PMCID: PMC3147906, PMID: 21632770]
- Kang HJ, Kadjo B, Dubey S, Jacquet F, Yanagihara R. Molecular evolution of Azagny virus, a newfound hantavirus harbored by the West African pygmy shrew (Crocidura obscurior) in Cote d’Ivoire. Virology Journal 2011 Jul 28;8:373. [PMCID: PMC3163557, PMID: 21798050]
- Kang HJ, Kosoy MY, Shrestha SK, Shrestha MP, Pavlin JA, Gibbons RV, Yanagihara R. Short report: genetic diversity of Thottapalayam virus, a hantavirus harbored by the Asian house shrew (Suncus murinus) in Nepal. American Journal of Tropical Medicine and Hygiene 2011 Sep;85(3):540-5. [PMCID: PMC3163881, PMID: 21896819]
- Kang Y, Norris MH, Wilcox BA, Tuanyok A, Keim PS, Hoang TT. Knockout and pullout recombineering for naturally transformable Burkholderia thailandensis and Burkholderia pseudomallei. Nature Protocols 2011 Jul 7;6(8):1085-104. [PMID: 21738123]
- Kelley JF, Kaufusi PH, Nerurkar VR. Dengue hemorrhagic fever-associated immunomediators induced via maturation of dengue virus nonstructural 4B protein in monocytes modulate endothelial cell adhesion molecules and human microvascular endothelial cells permeability. Virology 2012 Jan 20;422(2):326-37. [PMCID: PMC3273497, PMID: 22129847]
- Kelley JF, Kaufusi PH, Volper EM, Nerurkar VR. Maturation of dengue virus nonstructural protein 4B in monocytes enhances production of dengue hemorrhagic fever-associated chemokines and cytokines. Virology 2011 Sep 15;418(1):27-39. [PMCID: PMC3184475, PMID: 21810535]
- Norris MH, Propst KL, Kang Y, Dow SW, Schweizer HP, Hoang TT. (2011) The Burkholderia pseudomallei delta asd mutant exhibits attenuated intracellular infectivity and imparts protection against acute inhalation melioidosis in mice. Infection and Immunity 2011 Oct;79(10):4010-8. [PMCID: PMC3187240, PMID: 21807903]
- Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. Journal of General Virology 2012 Mar 7. [PMID: 22398316]
- Sumibcay L, Kadjo B, Gu SH, Kang HJ, Lim BK, Cook JA, Song JW, Yanagihara R. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Cote d’Ivoire. Virology Journal 2012 Jan 26;9(1):34. [PMID: 22281072]
- Yang B, Li HW. Dicer assay in Drosophila S2 cell extract. Methods in Molecular Biology 2011;721:215-29. [PMID: 21431688]
2010-2011
- de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Neglected Tropical Diseases 2011 Jun;5(6):e1188. [PMCID: PMC3119640, PMID: 21713020]
- Chen I, Kaufusi P, Erdem G. Emergence of erythromycin and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. Journal of Clinical Microbiology 2011 Jan;49(1):439-41. [PMCID: PMC3020461, PMID: 21068284]
Garcia AF, Abe LM, Erdem G, Cortez CL, Kurahara D, Yamaga K. An insert in the covS gene distinguishes a pharyngeal and a blood isolate of Streptococcus pyogenes found in the same individual. Microbiology 2010 Oct;156(Pt 10):3085-95. [PMCID: PMC3068697, PMID: 20634239]
Gu SH, Kang HJ, Baek LJ, Noh JY, Kim HC, Klein TA, Yanagihara R, Song JW. Genetic diversity of Imjin virus in the Ussuri white-toothed shrew (Crocidura lasiura) in the Republic of Korea, 2004-2010. Virology Journal 2011 Feb 8;8:56. [PMCID: PMC3046926, PMID: 21303516]
- Hsieh SC, Zou G, Tsai WY, Qing M, Chang GJ, Shi PY, Wang WK. The C-terminal helical domain of dengue virus precursor membrane protein is involved in virus assembly and entry. Virology 2011 Feb 5;410(1):170-80. [PMCID: PMC3100346, PMID: 21129763]
- Kang HJ, Arai S, Hope AG, Cook JA, Yanagihara R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus). Vector Borne and Zoonotic Diseases 2010 Aug;10(6):593-7. [PMCID: PMC2979330, PMID: 20426682]
Kumar M, Verma S, Nerurkar VR. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. Journal of Neuroinflammation 2010 Oct 31;7:73. [PMCID: PMC2984415, PMID: 21034511]
Lin SR, Zou G, Hsieh SC, Qing M, Tsai WY, Shi PY, Wang WK. The helical domains of the stem region of envelope protein of dengue virus are involved in virus assembly and entry. Journal of Virology 2011 May;85(10):5159-71. [PMCID: PMC3126166, PMID: 21367896]
Norris M, Kang Y, Wilcox BA, Hoang TT. Stable site-specific fluorescent tagging constructs optimized for Burkholderia species. Applied and Environmental Microbiology 2010 Nov;76(22):7635-40. [PMCID: PMC2976199, PMID: 20851961]
- Steel A, Gubler DJ, Bennett SN. Natural attenuation of dengue virus type-2 after a series of island outbreaks: a retrospective phylogenetic study of events in the South Pacific three decades ago. Virology 2010 Sep 30;405(2):505-12. [PMCID: PMC3150181, PMID: 20663532]
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. Journal of Immunology 2011 Feb 15;186(4):2127-37. [PMCID: PMC3088479, PMID: 21220695]
Verma S, Kumar M, Nerurkar VR. Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes. Journal of General Virology 2011 Mar;92(Pt 3):507-15. [PMCID: PMC3081232, PMID: 21106803]
Yashina LN, Abramov SA, Gutorov VV, Dupal TA, Krivopalov AV, Panov VV, Danchinova GA, Vinogradov VV, Luchnikova EM, Hay J, Kang HJ, Yanagihara R. Seewis virus: phylogeography of a shrew-borne hantavirus in Siberia, Russia. Vector Borne and Zoonotic Diseases 2010 Aug;10(6):585-91. [PMCID: PMC2979336, PMID: 20426688]
Zhang L, Strianese O, Gaudino G, Morris P, Pass HI, Nerurkar VR, Bocchetta M, Carbone M. Tissue tropism of SV40 transformation of human cells: role of the viral regulatory region and of cellular Notch-1. Genes and Cancer 2010 Oct;1(10):1008-20. [PMCID: PMC3092263, PMID: 21779427]
2009-2010
Artsob H, Gubler DJ, Enria DA, Morales MA, Pupo M, Bunning ML, Dudley JP. West Nile Virus in the new world: trends in the spread and proliferation of West Nile Virus in the western hemisphere. Zoonoses Public Health 2009 Aug;56(6-7):357-69. [PMID: 19486320]
Bennett SN, Drummond AJ, Kapan DD, Suchard MA, Munoz-Jordan JL, Pybus OG, Holmes EC, Gubler DJ. Epidemic dynamics revealed in dengue evolution. Molecular Biology and Evolution 2010 Apr;27(4):811-8. [PMCID: PMC2877535, PMID: 19965886]
Gubler DJ. Vector-borne diseases. Revue Scientifique et Technique 2009 Aug;28(2):583-8. [PMID: 20128467]
- Hsieh SC, Tsai WY, Wang WK. The length of and nonhydrophobic residues in the transmembrane domain of dengue virus envelope protein are critical for its retention and assembly in the endoplasmic reticulum. Journal of Virology 2010 May;84(9):4782-97. [PMCID: PMC2863728, PMID: 20181718]
Imrie A, Roche C, Zhao Z, Bennett S, Laille M, Effler P, Cao-Lormeau VM. Homology of complete genome sequences for dengue virus type-1, from dengue-fever- and dengue-haemorrhagic-fever-associated epidemics in Hawaii and French Polynesia. Annals Tropical Medicine and Parasitology 2010 Apr;104(3):225-35. [PMCID: PMC3084289, PMID: 20507696]
Kang HJ, Bennett SN, Sumibcay L, Arai S, Hope AG, Mocz G, Song JW, Cook JA, Yanagihara R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS One 2009 Jul 7;4(7):e6149. [PMCID: PMC2702001, PMID: 19582155]
Kang HJ, Arai S, Hope AG, Song JW, Cook JA, Yanagihara R. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virology Journal 2009 Nov 24;6:208. [PMCID: PMC2789066, PMID: 19930716]
- Lieberman MM, Nerurkar VR, Luo H, Cropp B, Carrion R, Jr., de la Garza M, Coller BA, Clements D, Ogata S, Wong T, Martyak T, Weeks-Levy C. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clinical and Vaccine Immunology 2009 Sep;16(9):1332-7. [PMCID: PMC2745014, PMID: 19641099]
Rai MA, Nerurkar VR, Khoja S, Khan S, Yanagihara R, Rehman A, Kazmi, SU, Syed HA. Evidence for a founder effect among HIV-1-infected injection drug users (IDUs) in Pakistan. BMC Infectious Diseases 2010 Jan 12;10:7. [PMCID: PMC2820481, PMID: 20064274]
Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010 Feb 5;397(1):130-8. [PMCID: PMC3102050, PMID: 19922973]
Links
Contact Us
Director:Vivek R. Nerurkar, Ph.D.Email: nerurkar [at] pbrc.hawaii.edu Associate Director:Mukesh Kumar, M.S., Ph.D.Email: mukesh [at] hawaii.edu |
|